Compare the Composition of Binary Ionic and Binary Molecular
The word ion is dropped from both parts. This lesson will reveal the rules for writing the names and chemical formulae of ionic compounds by balancing charges and using the cross method.
Molecules Ions And Chemical Formulas
We review their content and use your feedback to keep the quality high.

. This problem has been solved. This is the main difference between ionic and molecular compounds. Side by Side Comparison Binary vs Ternary Acids in Tabular Form 6.
Students will be able to transition between chemical names and chemical formulas of binary ionic compounds and binary covalent compounds. 1 for the compound BaCl 2. Covalent or Molecular compounds.
Identify the following as binary ionic or binary covalent compund. Here is a link that describes binary ionic compounds how does this difffer from what you. Solvation dynamics in room temperature ionic liquids.
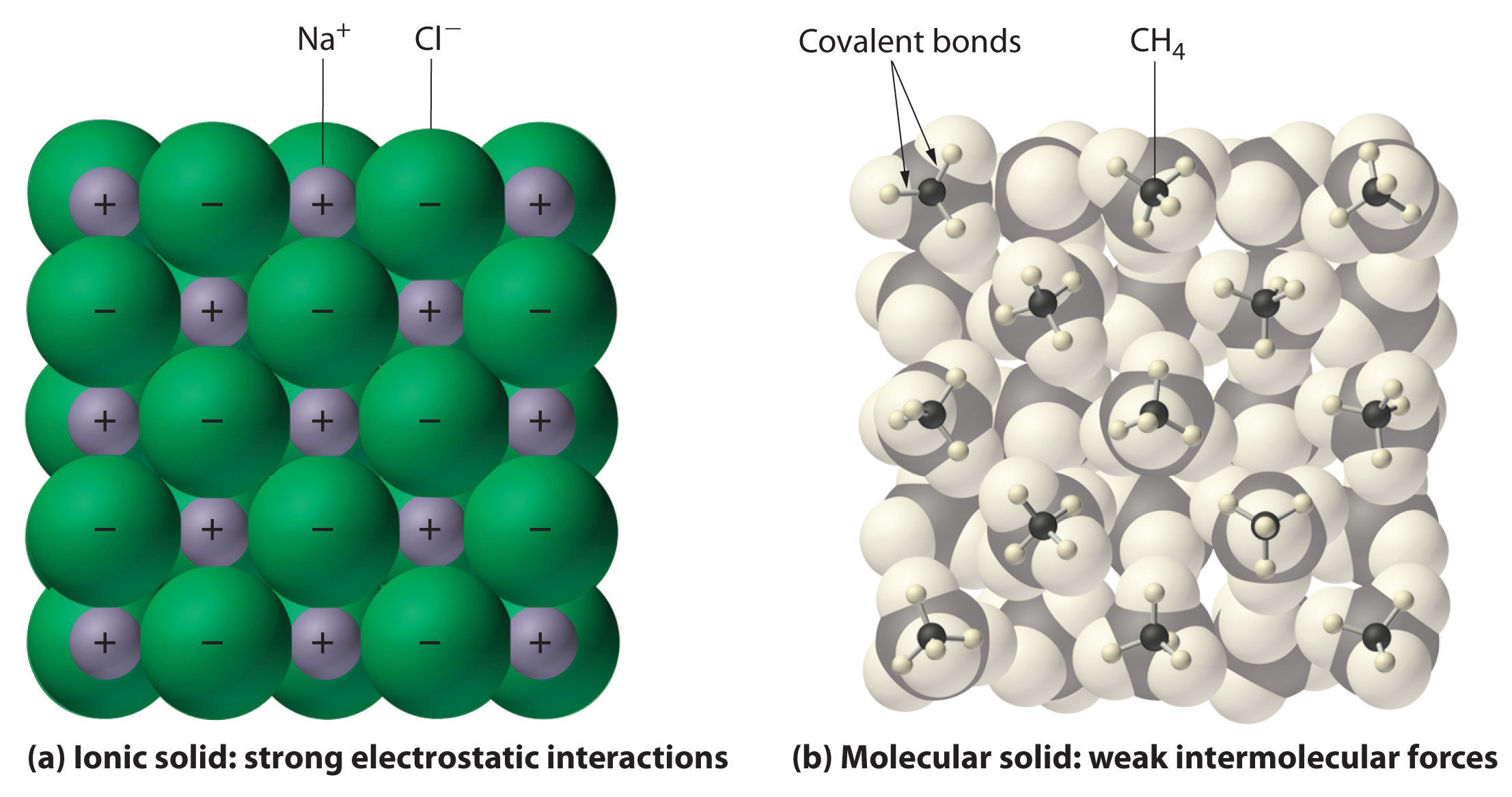
A binary molecular compound will form molecules for example water H2O carbon. Ionic compounds are made of ionic bonds and molecular compounds are made of covalent bonds. Characterize both molecular structure features and.
Called a covalent bond. Binary Ionic Compounds Binary Compounds 2 elements. The metal cation is named first followed by the nonmetal anion as illustrated in Figure 45.
A polyatomic ion is a group of atoms bond by covalent bonds with an unbalanced charge. The name of an oxyacid consists of a form of the root of the anion a suffix and the word acid. To write the names just name the two ions.
CCl4 CaI2 MgO NaCl PI3 Cal2 SF4 LiN3 BaO BF3 H2O2 SrCl2 Rb3N Ba2N3 RaF2 Who are the experts. Then either give the same or chemical formula as necessary for each problem below. Dynamic Stokes shift studies of fluorescence of dipolar molecules By Prasun Mandal and Satyen Saha Stokes shift dynamics of ionic liquids.
A cation and an anion. All ionic compounds have numerous properties in common. Students will be able to predict formulas for stable binary ionic compounds based on balance of charges.
Polyatomc ions are charged compounds composed of non-metals with covalent bonds created by gaining or losing electrons to form the bonds. First quickly scan the worksheet and circle any metals as a symbol or as a name that is a transition metal. Naming Binary Ionic Compounds - Properties and Structure of Matter.
Experts are tested by Chegg as specialists in their subject area. A binary acid is an acidic compound that always has hydrogen boned to another chemical elements most of the times a nonmetal. Binary Ionic Compounds and Their Properties.
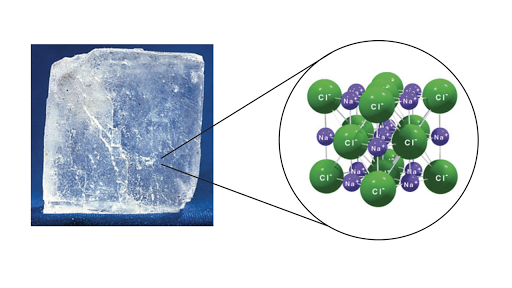
A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion. An ionic compound will contain cations and anions and form an infinite lattice for example sodium chloride NaCl 2. It is called binary because it has two different elements combined with each other.
Molecular compounds contain only non-metals carbon hydrogenphosphorus while ionic compounds contain at least one metalzinc manganese iron and at least one non-metal. A shared pair of e and anions atoms that have gained e-. Consequently the ability to recognize an ionic compound from its formula will allow you to predict many of its properties.
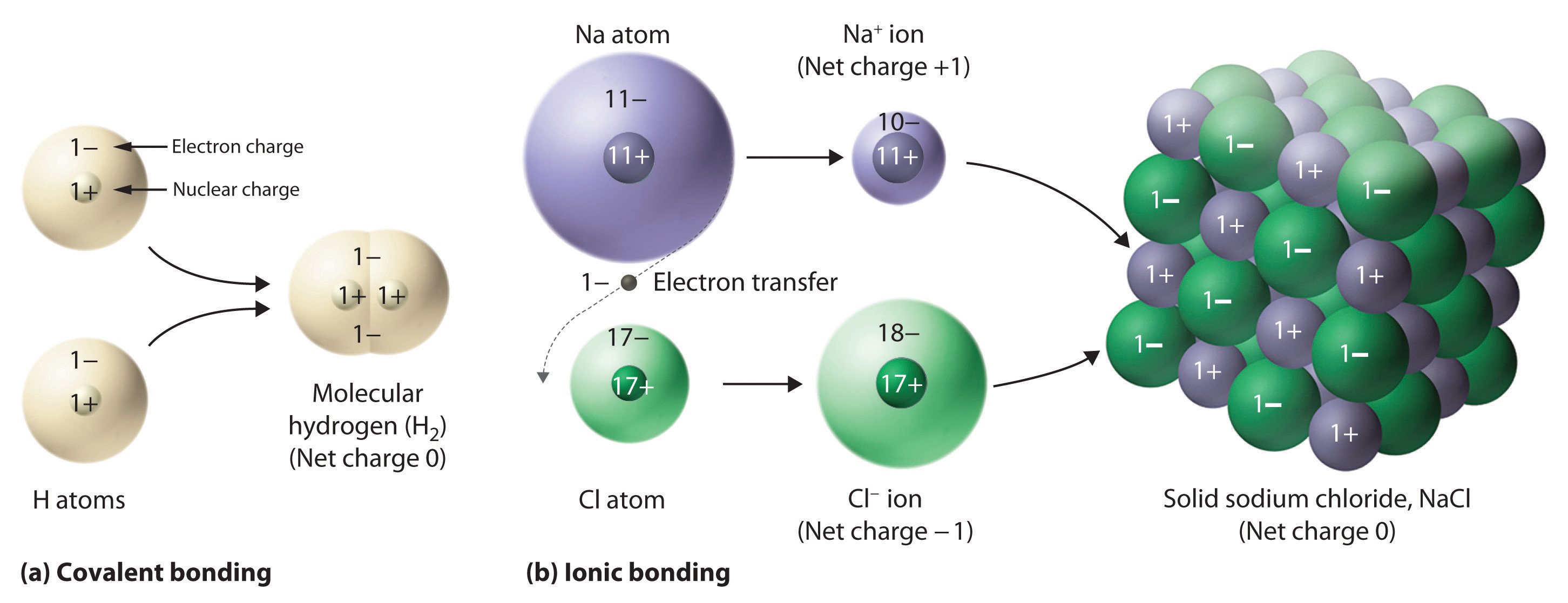
What are Binary Acids. A binary ionic compound is composed of atoms of two elements bond by an ionic bond. Compare and contrast ionic and covalent bonds in terms of electron movement.
Remember that transition metals can have multiple oxidation states so you are required. The rest of the name consists of a form of the root of the second element plus the suffix -ic followed by the word acid. This is often possible in the case of a binary compound one which contains only two elements because formation of.
But we are not here to do your homework for you you will not learn it that way. 1 Naming B a C l 2. Are formed by sharing of electron pairs in -is.
Ionic bonds occur between two species which are electrostatically attracted towards each other whereas covalent bonds from through the sharing of electrons between their outer shells. If the suffix is -ite change it to. Are formed by electrostatic attraction between cations atoms that have lost e- between atoms.
Easy with Representative elements Groups 1 2 13 NaCl Na Cl- sodium chloride MgBr 2 Mg2 Br- magnesium bromide. When naming a binary acid use the prefix hydro- to name the hydrogen part of the compound. Solute probe dependence and effects of self-motion dielectric relaxation frequency window and collective intermolecular solvent modes.
Students will understand how electrons are involved in ionic and covalent bonding. Predicting Thermal Decomposition Temperature of Binary Imidazolium Ionic Liquid Mixtures from Molecular Structures. Binary Ionic Compounds ANSWER KEY.
If the anion suffix is -ate replace it with -ic. Tell us what you come up with and we will guide you from there. Compare the compositions of binary ionic and binary molecular compounds.
Predict formulas for stable binary ionic compounds based on balance of charges. Students will explore the nature of matter its classifications and its system for naming types of matter. This creates an unbalanced charge.

Difference Between Ionic And Binary Compounds Compare The Difference Between Similar Terms

Molecular And Ionic Compounds Chemistry For Majors

Naming Ionic Compounds Puzzle Sheet Teaching Chemistry Chemistry Classroom Chemistry Worksheets
Molecules Ions And Chemical Formulas

What Re The Differences Between Ionic Molecular Binary Compounds Quora

Chapter 7 Lesson Starter Ccl 4 Mgcl 2 What Kind Of Information Can You Discern From The Formulas Which Of The Compounds Represented Is Molecular And Ppt Download

Chemical Nomenclature Ppt Download
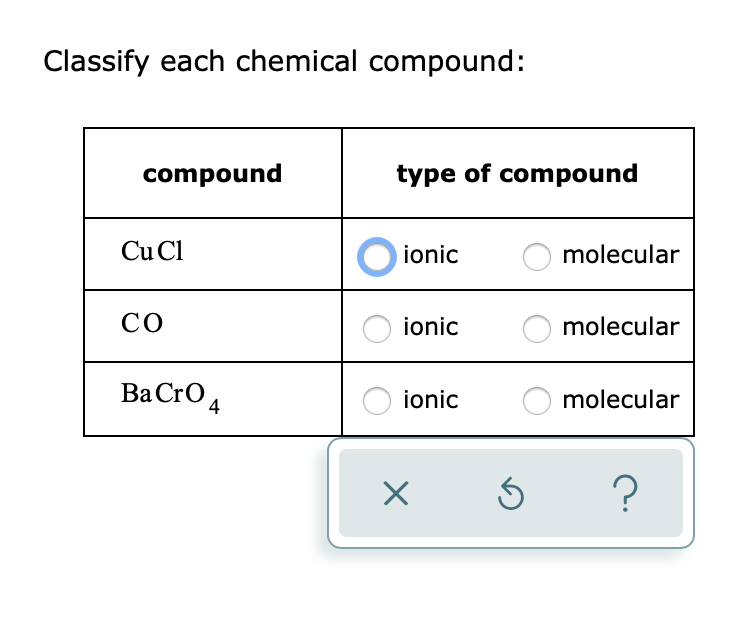
Solved Classify Each Chemical Compound Compound Type Of Chegg Com
Molecules And Compounds Overview Atomic Structure Article Khan Academy

Ap Chemistry Master Cheatsheet Ap Chemistry Chemistry Basics Chemistry Education
What S The Difference Between An Ionic Bond And A Covalent Bond Quora

Unit 4 Chapter 7 Writing Formulas Naming Compounds Ppt Download

Ionic And Molecular Compounds Infographic Misses Out Giant Covalent Yet Lists Sio2 As Covalent Examp Chemistry Classroom Teaching Chemistry Chemistry Education

Difference Between Ionic And Binary Compounds Compare The Difference Between Similar Terms

High School Chemistry Year Curriculum High School Chemistry Chemistry Curriculum

Chemical Bonding Chemistry Homework Page Unit Bundle Chemistry Lessons Chemistry Education Teaching Chemistry

Ionic Compound Speed Dating Formula Naming Practice Teaching Chemistry School Science Experiments Chemistry Lessons


Comments
Post a Comment